What Are Antibody–Drug Conjugates (ADCs)?
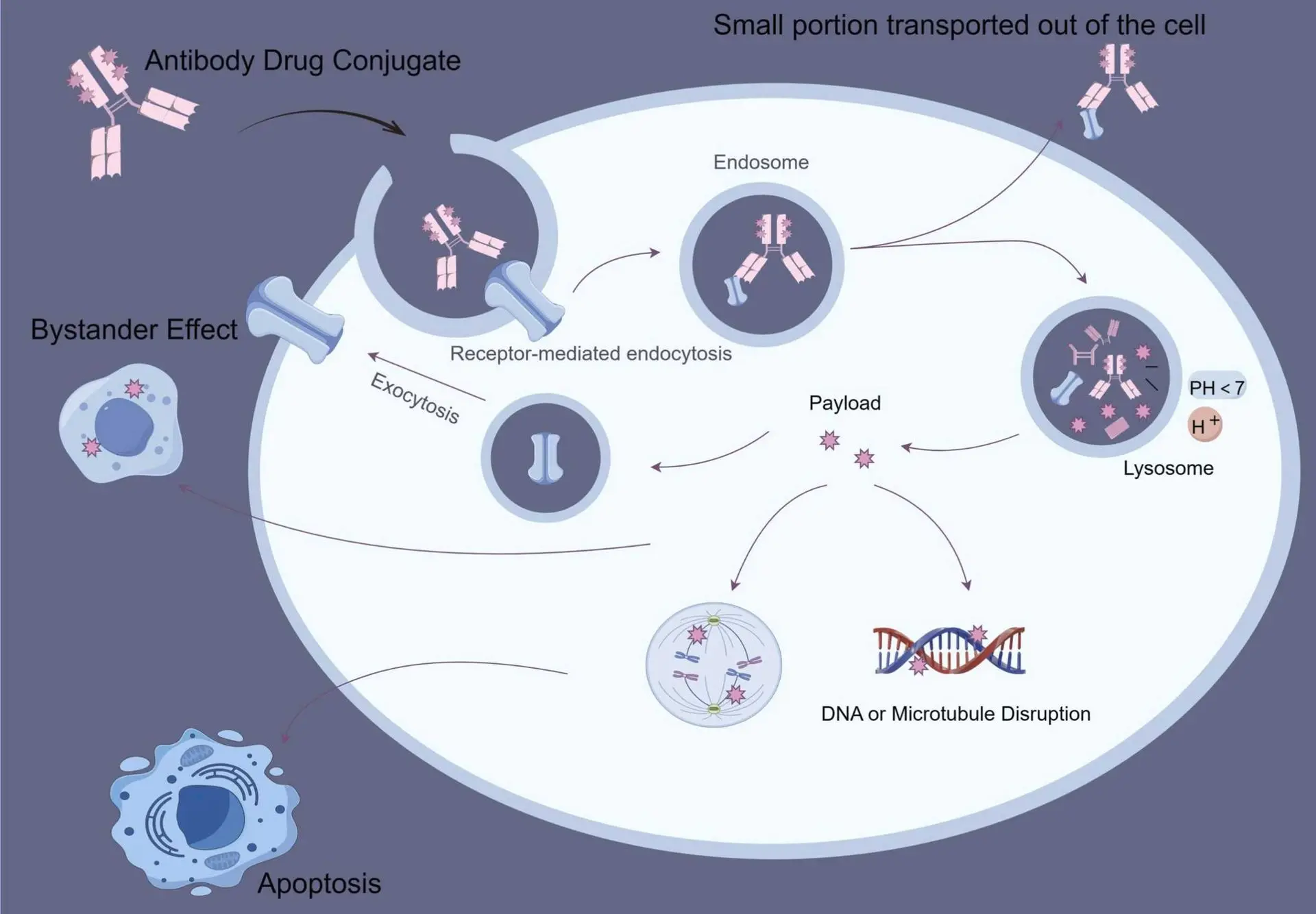
ADCs represent a cutting-edge class of targeted cancer therapies that merge the high specificity of monoclonal antibodies with the potent cytotoxic effects of chemotherapy agents. Designed to function like guided missiles, ADCs operate through a three-part mechanism. First, the antibody component selectively binds to specific antigens found on the surface of cancer cells. Next, a chemical linker securely holds the antibody and the cytotoxic payload together as the complex circulates through the body. Once the ADC reaches its target and is internalized by the cancer cell, the payload is released intracellularly to exert its lethal effect. This targeted delivery system enables ADCs to efficiently eliminate tumor cells while significantly reducing collateral damage to healthy tissue offering a level of precision that traditional chemotherapy cannot achieve.
The Mechanism of ADC
Redefining Cancer Therapy: How ADCs Are Advancing Precision Oncology
Antibody–Drug Conjugates (ADCs) embody the core principles of precision medicine by enabling the selective eradication of malignant cells while preserving healthy tissues. Unlike conventional chemotherapy, which circulates systemically and affects rapidly dividing cells indiscriminately, ADCs are designed to recognize tumor-specific antigens—such as HER2, CD30, or c-Met—expressed predominantly on the surface of cancer cells. By coupling these antibodies with powerful cytotoxic drugs via stable linkers, ADCs ensure that the therapeutic payload is delivered directly into the tumor microenvironment, limiting off-target toxicity. This targeted mechanism is particularly valuable in cancers with poor prognosis or resistance to standard therapies, such as triple-negative breast cancer, relapsed lymphoma, or metastatic non-small cell lung cancer. As tumor heterogeneity and drug resistance continue to challenge oncologists, the demand for therapies like ADCs that combine precision targeting with high efficacy is rapidly growing redefining how clinicians approach complex cancers.
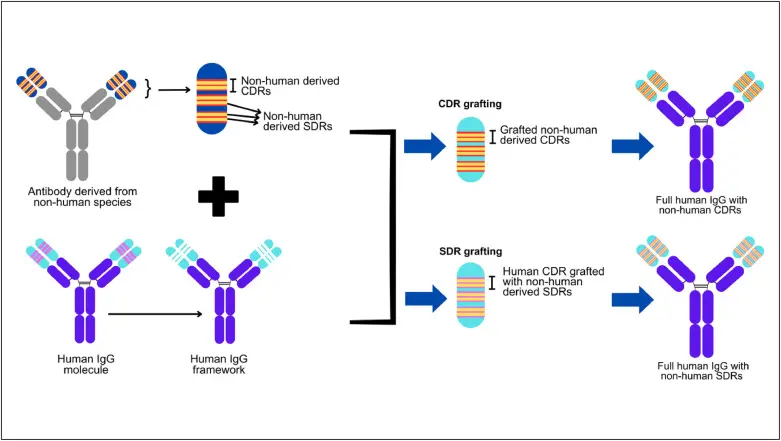
CDR grafting and SDR grafting method in antibody humanization.
Antigen Specificity: Leveraging Tumor-Selective Expression
The therapeutic efficacy of Antibody–Drug Conjugates (ADCs) is fundamentally dependent on the precise identification of tumor-associated antigens (TAAs). These cell surface molecules, such as HER2, CD30, Trop-2, or c-Met, are often overexpressed in malignant tissues while showing limited or no expression in normal cells. Monoclonal antibodies within the ADC structure are engineered to exploit this differential expression, allowing high-affinity, antigen-specific binding that minimizes off-target interactions. Upon antigen recognition, receptor-mediated endocytosis is initiated, enabling the internalization of the ADC-antigen complex into the tumor cell. The choice of antigen is critical not only for targeting efficacy but also for ensuring rapid internalization and lysosomal trafficking, which are prerequisites for successful payload release. This biological selectivity underpins the ability of ADCs to achieve high therapeutic indices, even in tumors refractory to standard cytotoxic regimens.
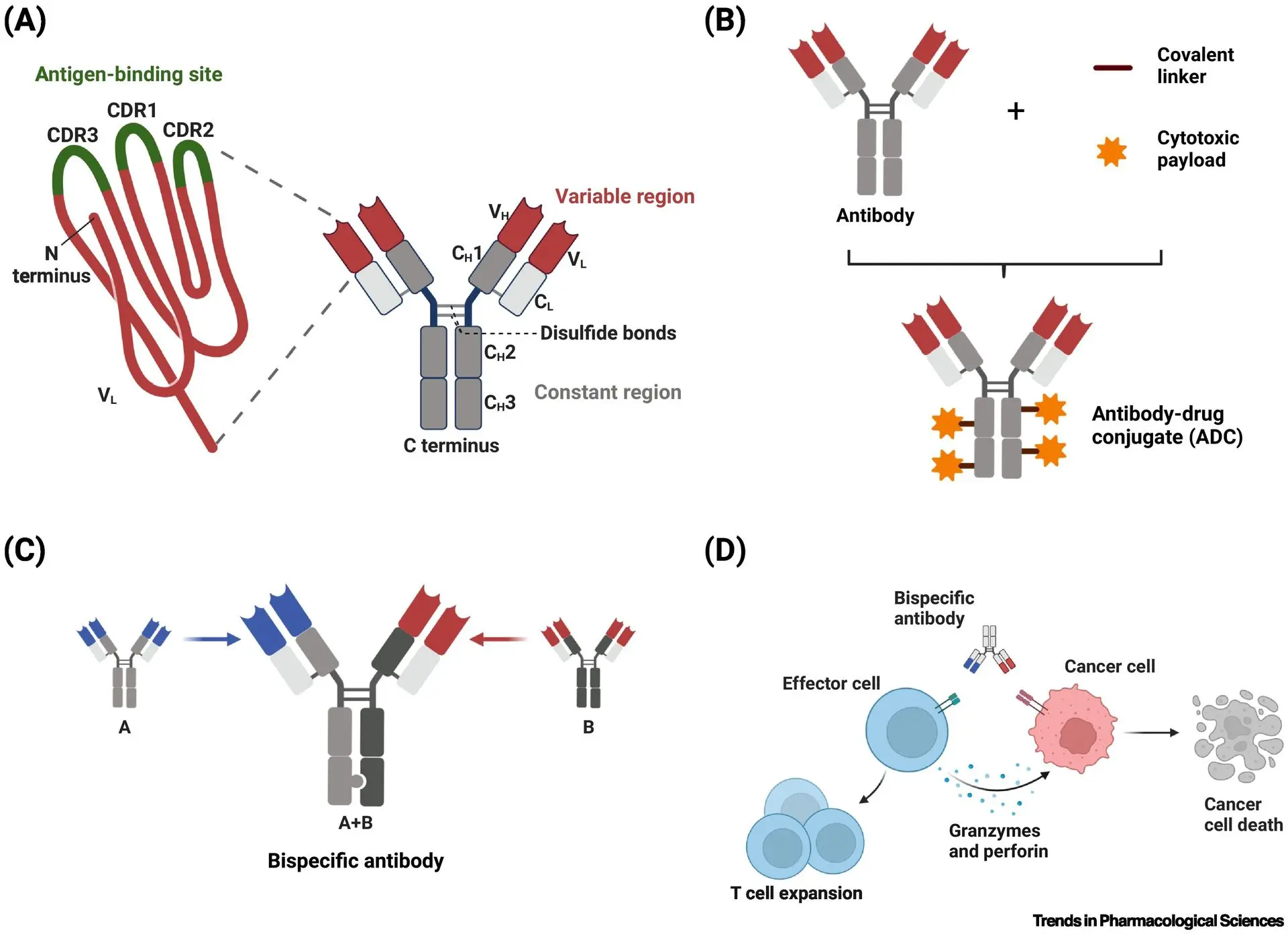
Common antibody-based therapeutic formats
(A) monoclonal antibody, (B) antibody–drug conjugate, (C) bispecific antibody, and (D) T cell engager.
Linker Chemistry: The Molecular Gatekeeper of Payload Activation
The linker plays a central role in the pharmacokinetics, safety, and efficacy of ADCs by acting as a conditional release mechanism for the cytotoxic payload. It must remain chemically stable in systemic circulation to prevent premature drug liberation and associated toxicity, yet be cleavable under intracellular tumor-specific conditions. There are two major classes of linkers: cleavable linkers (enzyme-sensitive, acid-sensitive, or glutathione-responsive) and non-cleavable linkers that rely on complete proteolytic degradation of the antibody within lysosomes. For instance, valine–citrulline dipeptide linkers are widely used in clinically approved ADCs due to their sensitivity to cathepsin B, a protease upregulated in many tumors. Optimizing linker design influences not only the release kinetics but also the bystander killing effect, where released drug diffuses into adjacent tumor cells an especially beneficial feature in heterogeneous tumors with variable antigen density. As such, linker chemistry remains a key determinant of ADC safety and therapeutic breadth.
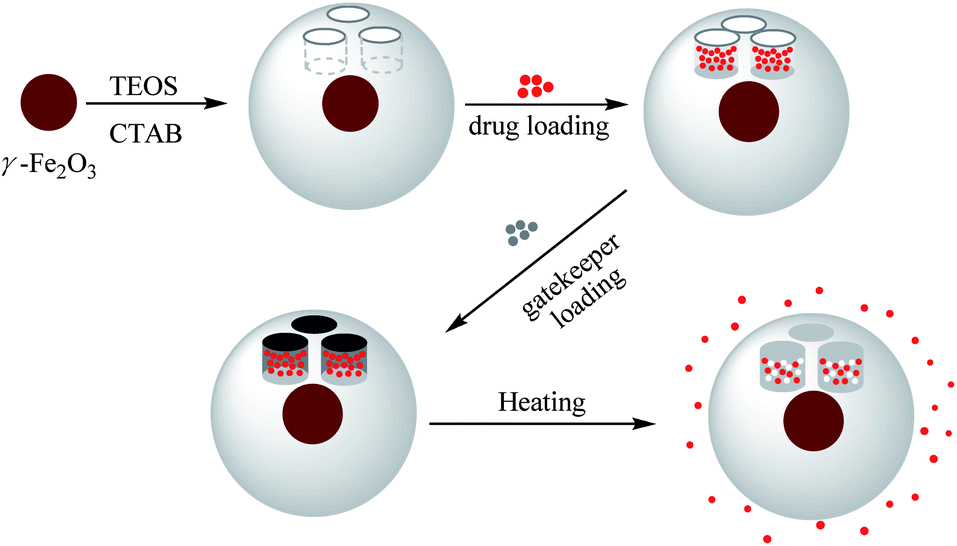
Preparation of γ-Fe2O3@MSNPs, drug loading of Doxorubicin®, immobilization with 1-tetradecanol as a gatekeeper, and triggered drug release by conventional external heating.
Cytotoxic Payloads: Maximizing Potency with Molecular Precision
The payload component of an Antibody–Drug Conjugate (ADC) is a highly potent cytotoxic agent, typically selected for its ability to induce irreversible cellular damage upon intracellular release. These agents function by targeting essential cellular processes such as microtubule dynamics, DNA replication, or topoisomerase activity, ultimately leading to cell cycle arrest and apoptosis. Due to their extreme potency, these compounds are generally unsuitable for systemic administration in free form but become therapeutically effective when precisely delivered via the targeted architecture of an ADC. Upon binding and internalization by the target cell, the payload is released into the cytoplasm usually through linker cleavage or lysosomal degradation where it exerts its lethal effect. Advanced ADC designs finely tune parameters such as drug–antibody ratio (DAR), linker stability, and intracellular trafficking efficiency to ensure optimal payload release, tumor penetration, and minimal systemic toxicity. This strategic delivery system enables ADCs to maximize therapeutic efficacy while preserving healthy tissues, making them a powerful tool in the treatment of aggressive and treatment-resistant malignancies.